Posted August 17, 2011 in Blog, Uncategorized, Xeomin
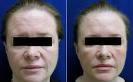
 Merz Pharmaceuticals today announced on August 2, 2011 that the United States (U.S.) Food and Drug Administration (FDA) has approved Xeomin botulinum toxin type A for the treatment of adults with cervical dystonia (stiff neck) or blepharospasm (twitching of the eyelids). According to an epidemiology study conducted in Rochester, Minnesota, the prevalence of focal dystonia, which includes cervical dystonia and blepharospasm, is estimated at 295 per million people in the U.S. Though 295 per million is a very small prevalence, in a totally unrelated epidemiology study casually conducted in Dr. Michael Persky’s Encino, California office, it was found that the prevalence of worrisome facial wrinkles in women over the age of 45 was 999,998 per million. Hence the popularity of neuromodulating injectable products such as Botox, Dysport, and now the third product FDA approved in the US, Xeomin.
Merz Pharmaceuticals today announced on August 2, 2011 that the United States (U.S.) Food and Drug Administration (FDA) has approved Xeomin botulinum toxin type A for the treatment of adults with cervical dystonia (stiff neck) or blepharospasm (twitching of the eyelids). According to an epidemiology study conducted in Rochester, Minnesota, the prevalence of focal dystonia, which includes cervical dystonia and blepharospasm, is estimated at 295 per million people in the U.S. Though 295 per million is a very small prevalence, in a totally unrelated epidemiology study casually conducted in Dr. Michael Persky’s Encino, California office, it was found that the prevalence of worrisome facial wrinkles in women over the age of 45 was 999,998 per million. Hence the popularity of neuromodulating injectable products such as Botox, Dysport, and now the third product FDA approved in the US, Xeomin.
Dr Michael Persky and Dr. Sarmela Sunder are located in Encino, California and Beverly Hills, California but service all of Los Angeles and the San Fernando Valley. Including, Beverly Hills, Hollywood, Hancock Park, Brentwood, Santa Monica, Pacific Palisades, Malibu, Sherman Oaks, Studio City, Calabasas, Woodland Hills, Tarzana, Westlake, Thousand Oaks, Agoura Hills and more.
XEOMIN is the only botulinum toxin that does not require refrigeration prior to reconstitution. Merz believes this may simplify product distribution and storage, and help ensure product integrity at the time of injection.. Botox is used similarly to Xeomin. If you have any questions or concerns regarding Xeomin and it’s use, feel free to contact us. Be well.